Periodic Patterns: Unraveling the Classification of Elements and Properties
Introduction: The Classification of Elements and Periodicity in Properties is a fundamental framework in chemistry, providing a systematic approach to understanding the diverse array of elements and their behavior. This classification, embodied in the periodic table, is a testament to the meticulous exploration of the relationships between elements and the predictable patterns that govern their properties.
Periodic Table: At the heart of this classification system is the periodic table, a visual representation that organizes elements based on their atomic number, electron configuration, and chemical properties. Rows, known as periods, and columns, known as groups or families, reveal trends and similarities among elements, offering a roadmap for understanding their behavior.
Periodicity in Atomic Properties: The periodic table exhibits periodicity in atomic properties, where elements in the same group share similar chemical characteristics. As one moves across a period from left to right, atomic size generally decreases, while moving down a group results in an increase in atomic size. These trends are attributed to changes in effective nuclear charge and electron shielding.
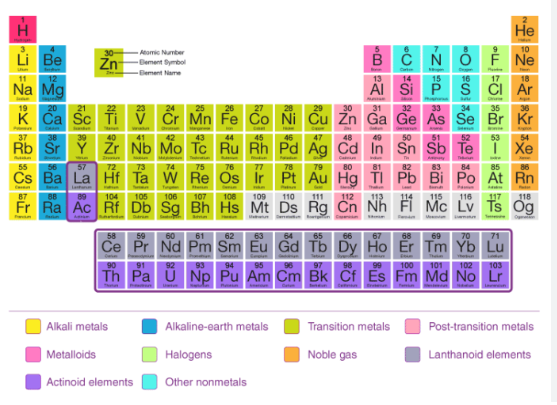
Chemical Reactivity and Periodicity: The arrangement of elements in the periodic table also correlates with their chemical reactivity. Noble gases, positioned in Group 18, exhibit inert behavior due to their complete electron shells, while alkali metals in Group 1 are highly reactive due to their tendency to lose electrons. Understanding these patterns enhances the prediction of an element’s reactivity and bonding tendencies.
Periodicity in Physical Properties: Beyond chemical reactivity, periodicity extends to physical properties such as melting points, boiling points, and ionization energies. Elements within the same group often display analogous physical characteristics, contributing to the predictability of their behavior in various chemical and physical contexts.
| Topic Name | |
|---|---|
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
