Demystifying the Elemental Blueprint: Exploring the Structure of Atoms
The structure of the atom, a cornerstone in the realm of chemistry, unfolds as a captivating journey through the fundamental building blocks of matter. At the heart of this exploration are subatomic particles—protons, neutrons, and electrons—orchestrating the intricate dance that defines an atom’s essence.
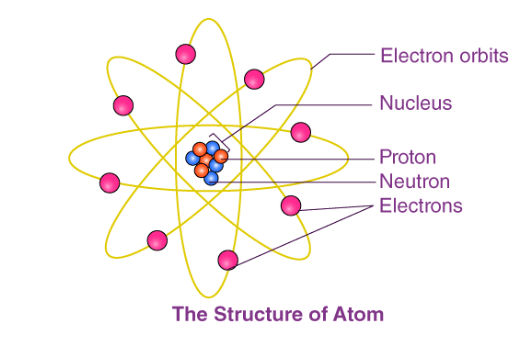
Subatomic Particles: Within the nucleus, protons carry a positive charge, while neutrons bear no charge. This central core, held together by the strong nuclear force, is surrounded by electrons, negatively charged particles orbiting in discrete electron shells. The arrangement of these particles forms the blueprint of atomic architecture.
Nucleus and Electron Shells: The nucleus, a minuscule yet dense entity, acts as the command center, housing protons and neutrons. Orbiting the nucleus are electron shells, akin to concentric layers, each with a designated energy level. Electrons, distributed across these shells, maintain specific energy states, dictating their behavior and interactions.
Atomic Number and Mass Number: The atomic number, a cardinal identifier denoted by Z, signifies the number of protons and uniquely defines an element. The mass number, denoted by A, encapsulates the sum of protons and neutrons within the nucleus. Isotopes, variations with identical atomic numbers but differing mass numbers, enrich the atomic landscape.
Electron Configuration: The dance of electrons within their designated shells follows the Aufbau principle, where lower energy levels are occupied before higher ones. The electron configuration, a roadmap of electron placement, underpins an atom’s chemical behavior and the manifestation of its distinct properties.
Quantum Mechanical Model: The quantum mechanical model, a pinnacle of atomic theory, transcends classical concepts, delving into the wave-particle duality of electrons. Probability distributions replace fixed orbits, offering a nuanced understanding of electron behavior. This model, rooted in quantum mechanics, reshapes our perception of the dynamic microcosm within each atom.
| Topic Name | |
|---|---|
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
Class 11 |
Download |
